Protein scaffolds for biocompatibility and increased sensitivity per agent
Cryptophane-based agents alone suffer from poor aqueous solubility and are limited by the fact that only one xenon atom can be associated with one cage molecule at any given time. This is a problem for in vivo applications which require solubility and maximum sensitivity—the detection threshold is too high to be broadly useful for the purposes of medical diagnostic imaging, where a central goal is to detect tumors, determine phenotype, and assess malignancy at an early stage. The refinement of targeted contrast agents is crucial for progress in molecular imaging.
In collaboration with the Francis and Wemmer groups, we have recently developed engineered nanoparticles that combine cryptophane molecular cages with protein scaffolds derived from viruses. By covalently attaching multiple cryptophanes to specific residues on the viruses, we can create a single agent that possesses the sensitivity of hundreds or even thousands of single cryptophane-based agents, and inherits the aqueous solubility and biocompatibility of the viral particle. To date, we have investigated this approach with MS2 viral capsids, as well as M13 bacteriophages.
MS2 is a viral capsid of nearly spherical structure with icosahedral symmetry composed of 180 monomers. After expression in E. coli, the coat protein monomers spontaneously assemble into genome-free, noninfectious capsids. Each capsid contains 32 pores, each ~2 nm wide, that facilitate access to the hollow interior. The interior and exterior surfaces of the capsid can be independently modified, enabling the simultaneous integration of cryptophane cages in the interior and cell-specific targeting units on the exterior. Previous reports have shown that MS2 viral capsids modified with nucleotide aptamers bind to specific cell receptors and are taken up by the cell
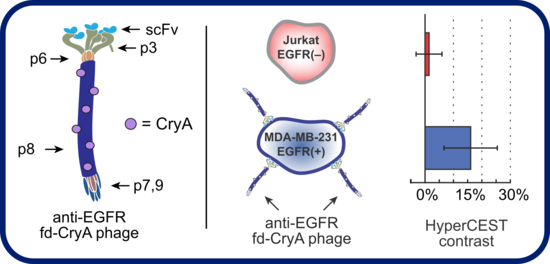
M13 is a filamentous bacteriophage approximately 1 micron in length and 10 nm in diameter. Like MS2, the phage is noninfectious to humans. It is principally composed of p8 coat protein monomers (red in the figure above), which are selectively PEGylated to increase hydrodynamic radius and subsequently modified to introduce cryptophane. M13 is versatile platform for creating targeted agents as it allows for phage display of ligands on p8 (red) coat proteins for high-valency display, as well as p3 (olive green) and p9 (dark blue) proteins for low-valency display. Importantly, it has been demonstrated that PEGylation of M13 has no detrimental effect on cellular uptake of the phage, and we have found no loss of sensitivity for cryptophane attached to M13 versus free cryptophane.
