Overview
Magnetic resonance (MR) spectroscopy and imaging has been used to determine structure, spatial distribution, and functional information of molecules. However, conventional NMR is limited in its ability to detect very low concentrations of material(1). This has restricted its utility as a reliable method for chemical assays and medical imaging where analytes or biomarkers are present in small quantities. The Pines Lab, in collaboration with Profs. David Wemmer and Matthew Francis, has developed sensors that utilize xenon atoms, rather than protons, as the source of signal for the NMR experiment. Xenon is an inert, non-toxic gas not naturally found in living organisms, whose signal slowly relaxes and can be dramatically enhanced (“hyperpolarized”) via laser-polarization such that small Xe concentrations can yield NMR signal comparable to that of water(2,3). This molecular sensor can be applied to a variety of applications and combined with other techniques such as remote detection and microfluidics to further enhance the specificity and detection limit of this technique.
Background
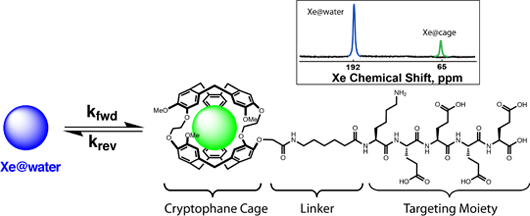
Molecular sensors work by generating a signal based on an interaction between molecular targets of interest and some binding motif typically a peptide. This technique is useful for complex chemical mixtures where we want to select out certain molecular targets, such as identifying chemical pollutants in environmental samples or contaminants in food grade samples. Currently a Xe atom in a cryptophane cage will be linked to a binding peptide and upon binding of the target molecule the MR spectrum of the Xe will shift confirming the presence of that molecular target. By hyperpolarizing the Xe before detection we can obtain a greatly increased signal(4).
History
The development of molecular sensors using fluorescence, mass spectrometry, and electrochemical techniques has been around for many years(5-8). Recently Xenon based molecular sensors have made MR an ideal platform to continue to advance the field. Work involving protein scaffolds have dramatically increased the amount of sensors that can be associated with a single molecular interaction, and the development of contrast agents that take advantage of relaxation properties for improved signal(9-12).
Sensitivity is further enhanced by combining the hyperpolarization step with chemical exchange saturation transfer (hyperCEST), which exploits the exchange of dissolved 129Xe nuclei between bulk solution (Xe@water) and cryptophane “molecular cages” (Xe@cryptophane)(13,14). The cage environment produces a chemical shift ~130 ppm upfield from that of Xe@water. A narrow-bandwidth radiofrequency pulse is applied at the chemical shift of Xe@cryptophane, saturating those spins without affecting the bulk pool of dissolved Xe. If the pulse duration is long relative to the mean residence time of Xe inside cryptophane (~10-3 – 10-2 s) saturation accumulates in the Xe@water pool due to the exchange of spins, resulting in a decrease in signal intensity that can easily be detected or imaged(15-17).
Current Work
Currently the group is working on coupling the Xe molecular sensor to microfluidic devices and optimizing the relaxation properties. These projects hope to improve sensitivity and eventually be coupled to projects in Ultra-Low Field NMR and Laser Enhanced NMR.
Current Members
Matt Ramirez
Daniel Kennedy
Clancy Slack
Phuong Dao
Keunhong Jeong
Muller Gomes
Jinny Sun
Collaborators
Dave Wemmer (UC-Berkeley, Chemistry)
Matt Francis (UC-Berkeley, Chemistry)
References
[1] H. Lee, T. Yoon, and R. Weissleder, Ultrasensitive detection of bacteria using core-shell nanoparticles and a NMR-filter system. Angew. Chem. Int. Ed. 48 (2009) 5657–5660.
[2] T.G. Walker, and W. Happer, Spin-exchange optical pumping of noble-gas nuclei. Reviews of Modern Physics 69 (1997) 629.
[3] I.C. Ruset, S. Ketel, and F.W. Hersman, Optical Pumping System Design for Large Production of Hyperpolarized Xe129. Phys Rev Lett. 96 (2006) 053002.
[4] K.W. Miller, N.V. Reo, A.J.M. Schoot Uiterkamp, D.P. Stengle, T.R. Stengle, and K.L. Williamson, Xenon NMR: Chemical Shifts of a General Anesthetic in Common Solvents, Proteins, and Membranes. PNAS 78 (1981) 4946–4949.
[5] Persaud, K. & Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 299, 352-355 (1982).
[6] Lamagna, A., Reich, S., Rodriguez, D., Boselli, A. & Cicerone, D. The use of an electronic nose to characterize emissions from a highly polluted river. Sensors and Actuators B-Chemical 131, 121-124 (2008).
[7] Giordani, D. S., Siqueira, A. F., Silva, M. L. C. P., Oliveira, P. C. & de Castro, H. F. Identification of the biodiesel source using an electronic nose. Energy & Fuels 22, 2743-2747 (2008).
[8] Ahn, S. M. & Simpson, R. J. Body fluid proteomics: Prospects for biomarker discovery. Proteomics Clinical Applications 1, 1004-1015 (2007).
[9] C. Hilty, T.J. Lowery, D.E. Wemmer, and A. Pines, Spectrally Resolved Magnetic Resonance Imaging of a Xenon Biosensor. Angew. Chem. Int. Ed. 45 (2006) 70–73.
[10] M.M. Spence, E.J. Ruiz, S.M. Rubin, T.J. Lowery, N. Winssinger, P.G. Schultz, D.E. Wemmer, and A. Pines, Development of a Functionalized Xenon Biosensor. J. Am. Chem. Soc. 126 (2004) 15267–15294.
[11] G. Huber, T. Brotin, L. Dubois, H. Desvaux, J.-P. Dutasta, and P. Berthault, Water Soluble Cryptophanes Showing Unprecedented Affinity for Xenon: Candidates as NMR-Based Biosensors. J. Am. Chem. Soc. 128 (2006) 6239–6246.
[12] L. Schröder, T.J. Lowery, C. Hilty, D.E. Wemmer, and A. Pines, Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science 314 (2006) 446449.
[13] M. Woods, D. Woessner, and A. Sherry, Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem. Soc. Rev. 35 (2006) 500–511.
[14] M.M. Spence, S.M. Rubin, I.E. Dimitrov, E.J. Ruiz, D.E. Wemmer, A. Pines, S.Q. Yao, F. Tian, and P.G. Schultz, Functionalized xenon as a biosensor. PNAS 98 (2001) 10654–10657.
[15] L. Schröder, T.J. Lowery, C. Hilty, D.E. Wemmer, and A. Pines, Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science 314 (2006) 446449.
[16] Meldrum T., Seim K.L, Bajaj V.S, Palaniappan K.K, Wu W., Francis M.B, Wemmer D.E, Pines A. 2010. A Xenon-Based Molecular Sensor Assembled on an MS2 Viral Capsid Scaffold. Journal of the American Chemical Society. 132(17):5936
[17] Stevens T.K., Palaniappan K.K., Ramirez R.M., Francis M.B., Wemmer D.E., Pines A. 2013. HyperCEST detection of a 129Xe-based contrast agent composed of cryptophane-A molecular cages on a bacteriophage scaffold. Magnetic Resonance in Medicine. 69(5):1245-1252.
