In NMR, information about chemical identity, topology, structure, and organization is encoded in chemical shifts, dipolar couplings, and electron-mediated scalar (J) couplings. In the absence of magnetic fields, the chemical shift vanishes, leaving the system to evolve only under what would otherwise be second-order interactions. In isotropic liquids, J-couplings are the primary magnetic interaction at zero-field. Even in the absence of chemical shifts, the J-couplings provide useful information about molecular topology, bond and torsion angles, hybridization, and bond strength. The J-couplings thus incorporate sufficient specific chemical information to yield unique spectral fingerprints for different molecules and functional groups. However, because the spin system is strongly coupled in this regime, conventional high-field spectral analysis techniques cannot readily be used, necessitating the development of new theories for understanding the zero-field spectrum.
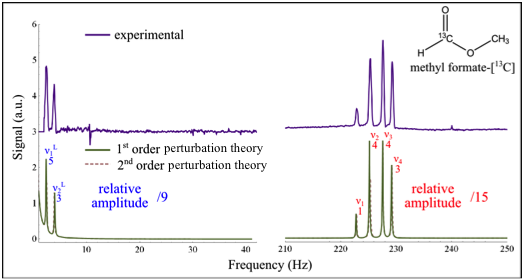 Because 1-bond J-couplings between heteronuclei are generally much stronger than longer-range couplings, we have developed a perturbative approach for the interpretation of J-spectra for simple spin systems. As illustrated by the figure at the left for the Zero-Field J-spectrum of methyl formate-13C, the spectrum predicted by first-order perturbation theory is typically a very good qualitative match, with higher order corrections just correcting slight shifts in frequency.
Because 1-bond J-couplings between heteronuclei are generally much stronger than longer-range couplings, we have developed a perturbative approach for the interpretation of J-spectra for simple spin systems. As illustrated by the figure at the left for the Zero-Field J-spectrum of methyl formate-13C, the spectrum predicted by first-order perturbation theory is typically a very good qualitative match, with higher order corrections just correcting slight shifts in frequency.
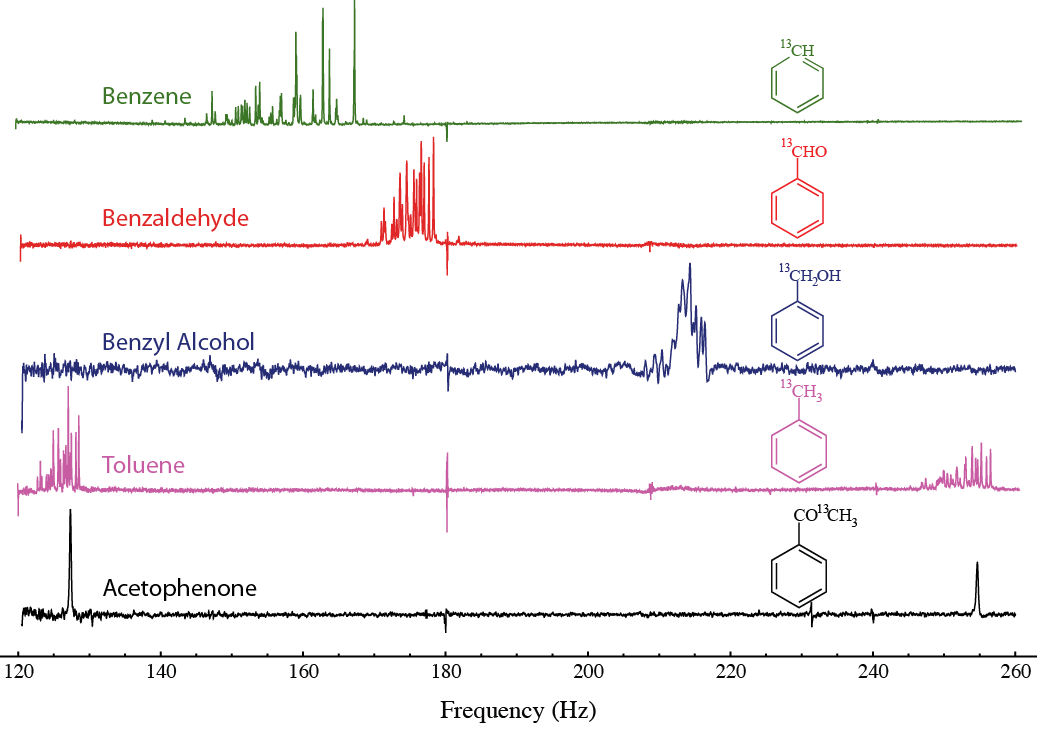
In order to demonstrate the viability of zero-field J-spectroscopy for chemical analysis, we have taken spectra for a series of labeled aromatic compounds, which give unique, understandable spectra, which are shown below. The zeroth-order spectral patterns do not overlap, which allows for straightforward determination of the spin interactions of substituent functional groups. Higher-order effects cause additional line splittings, revealing additional molecular information. We demonstrate resonance linewidths as narrow as 11 mHz, permitting resolution of minute frequency differences and precise determination of long-range J-couplings. Because the J-couplings are strongly dependent on electronic structure and molecular configuration, their precise measurement encourages the continued development of zero-field J-spectroscopy as a novel sensitive NMR technique for molecular identification and analysis.
Current Members
John Blanchard
Jonathan King
Tobias Sjolander
Collaborators
Dmitry Budker (UC Berkeley, Physics)
