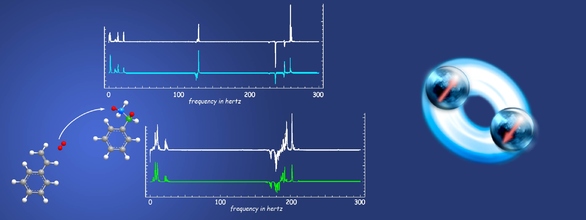
Despite the use of atomic magnetometers or superconducting quantum interference devices, low-field NMR using samples thermally prepolarized in a permanent magnet typically suffers from low signal-to-noise ratio compared with inductively detected high-field NMR, in part because of the low polarization available from thermalization in a permanent magnet. To avoid this difficulty, we have demonstrated large nuclear-spin polarization in zero-field NMR by employing the technique of parahydrogen-induced polarization (PHIP), whereby order from the singlet state of parahydrogen is transferred to a molecule of interest, either by hydrogenation or through reversible chemical exchange. When combined with sensitive atomic magnetometers for detection of nuclear spin magnetization, PHIP enables NMR without any magnets. The sensitivity is sufficient to easily observe complex spectra exhibiting 1H–13C (15N) J-couplings in compounds with 13C (15N) in natural abundance in just a few transients, a task that would require considerable signal averaging using thermal prepolarization. We have shown that polarization is efficiently transferred through many chemical bonds to remote parts of a molecule and to heteronuclei, because the Zeeman barrier to polarization transfer is absent at zero field. In the case of hydrogenative PHIP, we have further demonstrated spectral fingerprinting of different natural-abundance 13C isotopomers in ethylbenzene, the hydrogenation product of styrene. In non-hydrogenative PHIP (NH-PHIP), we have detected signals from samples with concentrations as low as 6 mM at zero field. We have also published theoretical work using symmetry arguments and simple model systems to describe the conversion of the singlet state of parahydrogen into observable oscillating magnetization at zero field.
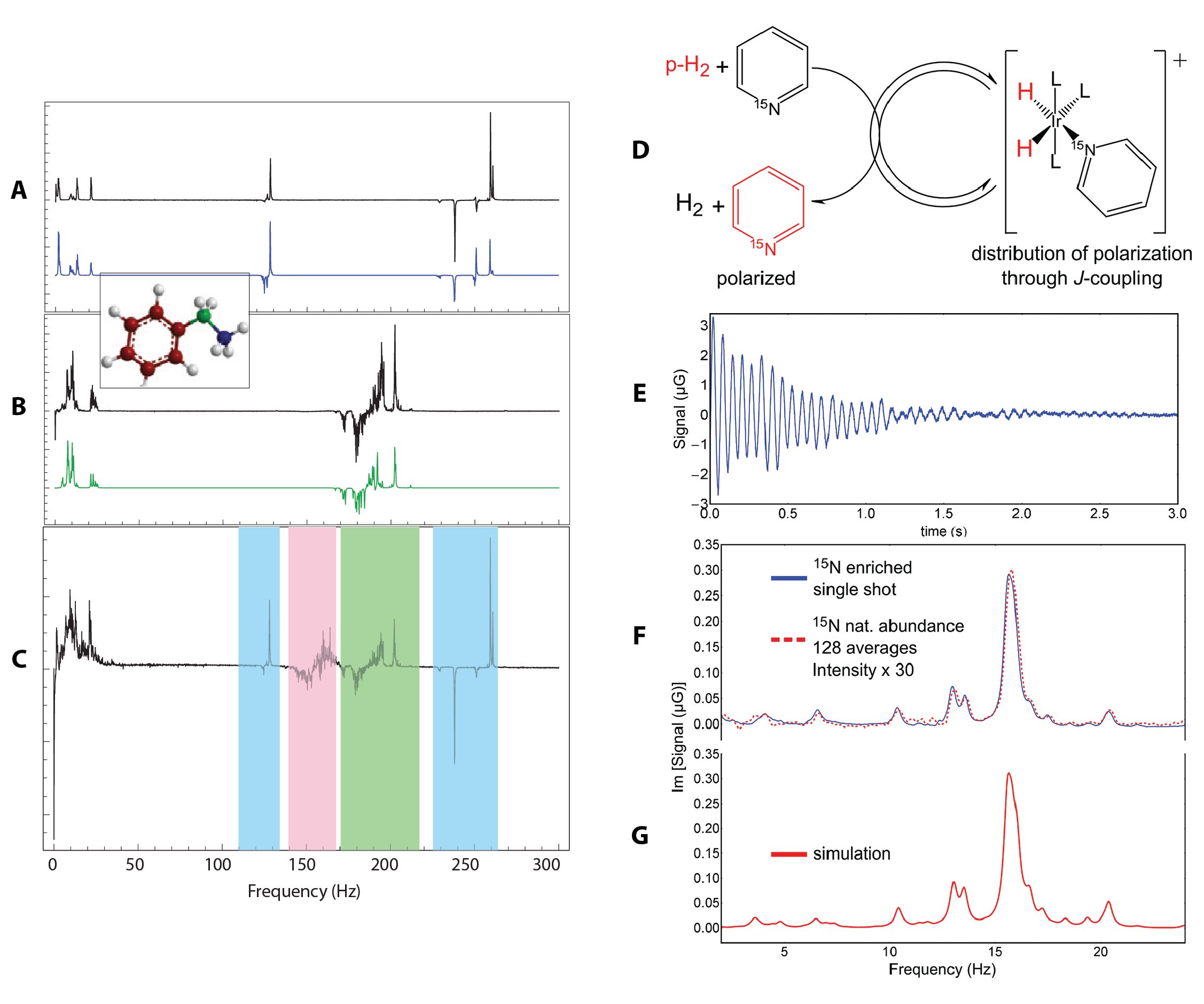
Figure 1. (A) and (B) show single-shot zero-field PHIP J-spectra for (A) ethylbenzene-β-13C and (B) ethylbenzene-α-13C polarized by the addition of parahydrogen to styrene. The blue and green are numerical simulations. (C) shows the zero-field spectrum for ethylbenzene with 13C in natural abundance from the averaging of eight transients. The high frequency components of the spectrum are easily recognizable from the α and β isotopomers and are highlighted by the green and blue bands, respectively. The signals highlighted in red are due to isotopomers with 13C on the benzene ring. (D) shows the NH-PHIP transfer mechanism, wherein parahydrogen and pyridine bind reversibly to the catalytic intermediate and polarization is transferred from parahydrogen through the J-coupling network. (E) is the single-shot transient, and (F) is the imaginary part of the Fourier transform for NH-PHIP polarized pyridine. In (F) the red dashed line is obtained from pyridine with 15N in natural abundance at a concentration of 3 M. (G) is a numerically simulated spectrum for comparison.
Current Members:
Danila Barskiy
Tobias Sjolander
Collaborators:
